The Shape of p. Helium has two electrons.
Therefore it can completely fill the 1s orbital with its two electrons.

. But for s orbitals l 0 so n - 1 n - l - 1 for s orbitals. Consider the difference between a 1s and 2s orbital. The 2s orbital can have more values of the ml quantum number than the 1s can.
The 1s orbital is always filled before any other orbital. 2p- orbitals are dumbbell shaped and oriented at right angles to each other. It would be the next closest orbital from the nucleus after 1s orbital.
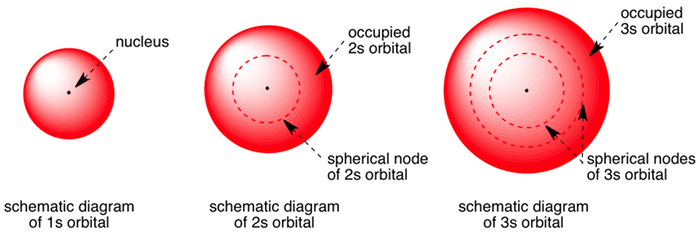
1s- orbital is spherical. The 2s has more spherical radial nodes. Same goes for the 3s orbital 4s etc.
Weve got the study and writing resources you need for your assignments. The major difference between a 1s orbital and a 2s orbital is that The 2s orbital has a slightly different shape. This is because the 2s orbital size resides farther away from the nucleus when compared to that of the 1s orbital.
Needs to be non-zero. The 2s has more spherical radial nodes. 1s and 2s orbitals belong to different energy levels.
Solution for How is a 2s orbital different from a 1s orbital. The 1s has fewer nuclear nodes. The 1s orbital has a higher energy than the 2s orbital.
The major difference between a 1s orbital and a 2s orbital is The 2s orbital is at a hue renege level An orbital that can never exist according to quantum description of the atom is. A 2s orbital has one more radial node. This is designated as 1s 2.
The radius of 2s orbital will be larger than that of 1s orbital. Draw a picture to illustrate the difference between the two. The 2s orbital is better at screening the outer orbitals than the 1s orbital.
1s and 2s are the sub-orbitals that are located in an atom. The size of the 2s orbital is larger than that of the 1s orbital. The 1s has fewer nuclear nodes.
The number of angular nodes is given by l the angular momentum quantum number so the number of radial nodes is n - l - 1. 2s orbital has higher energy than 1s. The whole ideadesign doesnt change really only the possible areas at which the Electron might be in is greater.
The 2s and 3p orbitals would have more nodes than 1s and 2p orbitalsWhat are two differences between a 2p and a 3p orbitalThe 3p orbitals have the same general shape. 1s and 2s orbitals occupied 2 electrons each and are spherical in nature. The 1s orbital is a sphere and the 2p orbital is made up of three dumbbells oriented in the x y and z direction.
Also the 2 s and 3 p orbitals would have more nodes. Start your trial now. The 2s orbital can hold more electrons.
Therefore since n increased by 1 2s orbitals have one more. Rutherford suggested the atom had a dense positively charged nucleus. My chemistry book explains that even though electrons in the mathrm2p orbital are closer to the nucleus on average electrons from the mathrm2s orbital spend a very short time very close to the nucleus penetration so it has a lower energy.
How Do The 2s And 3p Orbitals Differ From The 1s And 2p OrbitalsHow do the 2s and 3p orbitals differ from the 1s and 2p orbitals. The front lobes face away from each other and form a straight line leaving a 180 angle between the two orbitals. Why does this tiny amount of time spent close to the nucleus make such a big difference.
They are nearest to the nucleus and are found on the s sub-orbital. The 2s orbital would be the same shape as the 1s orbital but would be larger in size and the 3p orbital would have the same shape as the 2p orbitals bout would be larger in size. Therefore it has only one spot within the 1s orbital occupied.
The 2s orbital extends farther from the nucleus than the 1s. The 2s orbital extends farther from the nucleus than the 1s. The number of total nodes is n-1 where n is the principal quantum number n 1 2 3.
This is designated as 1s 1 where the superscripted 1 refers to the one electron within the 1s orbital. In which transition 1s 2s or 1s 2p is the charge density shifted the most. In it the 2s orbital and one of the 2p orbitals hybridize to form two sp orbitals each consisting of 50 s and 50 p character.
- Here n in the term ns determines the principal quantum number and. The 2s orbital is better at screening the outer orbitals than the 1s orbital. 2s- orbital is spherical with a diameter larger than that of the 1s orbital.
After 1s the electrons will fill us 2s orbital. Explain why the 1s 2s transition is not allowed whereas the 1s 2p transition is allowed in terms of the. 1s has low energy as compared to 2s.
The 2s orbital would be the same shape as the 1s orbital but would be smaller in size and the 3p orbitals would have a different shape than the 2p orbitals but would be larger in size. The 2s orbital is at a higher energy level. The s sub shell can hold a maximum of two electrons as there is only one orbital.
The 1s orbital has a higher energy than the 2s orbital. The 2s orbital can have more values of the ml quantum number than the 1s can. 2S orbital contains a nodal plane where 1s orbital does not have any node.
An illustration of the shape of the 1s 2s and 3s orbitals. This formation minimizes electron. The 1s orbital can have only one electron.
Describe Rutherfords model of the atom and compare it to the model of his student Niels Bohr. The 1 shows the orbital is within the energy level closest to the nucleus while the s describes the shape of the orbital spherical for S. First week only 499.
1s orbital has the lowest energy because it is located closed to the nucleus. S orbitals are spherical in shape and increase in size as the energy level or shell increases. Sp Hybridization can explain the linear structure in molecules.
In order for a transition between two states to occur the transition dipole. The difference between 1s and 2s is the difference in their level of energy. Hydrogen has one electron.

Difference Between 1s And 2s Orbital Compare The Difference Between Similar Terms
2s Orbital Contains 1s Orbital So If An Electron Is In 1s Is It Also In 2s How Do You Determine Quora
0 Comments